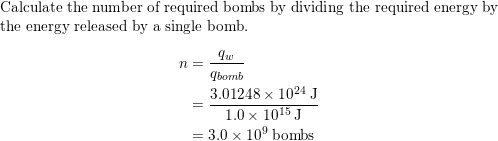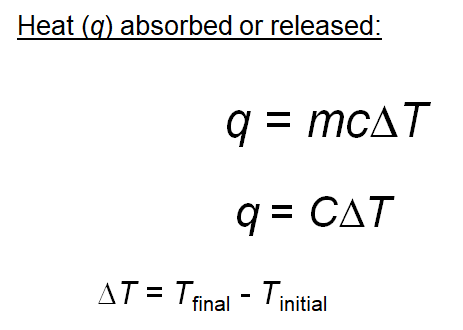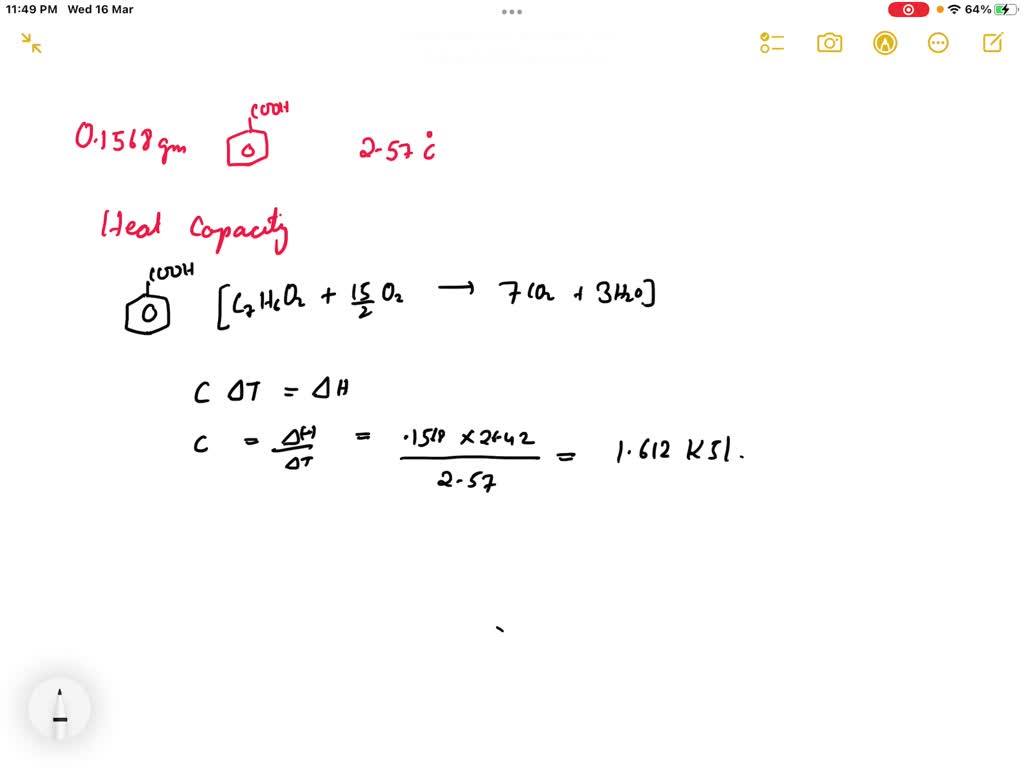
SOLVED: The combustion of 0.1568 g benzoic acid increases the temperature of a bomb calorimeter by 2.57°C. Calculate the heat capacity of this calorimeter. (The energy released by combustion of benzoic acid
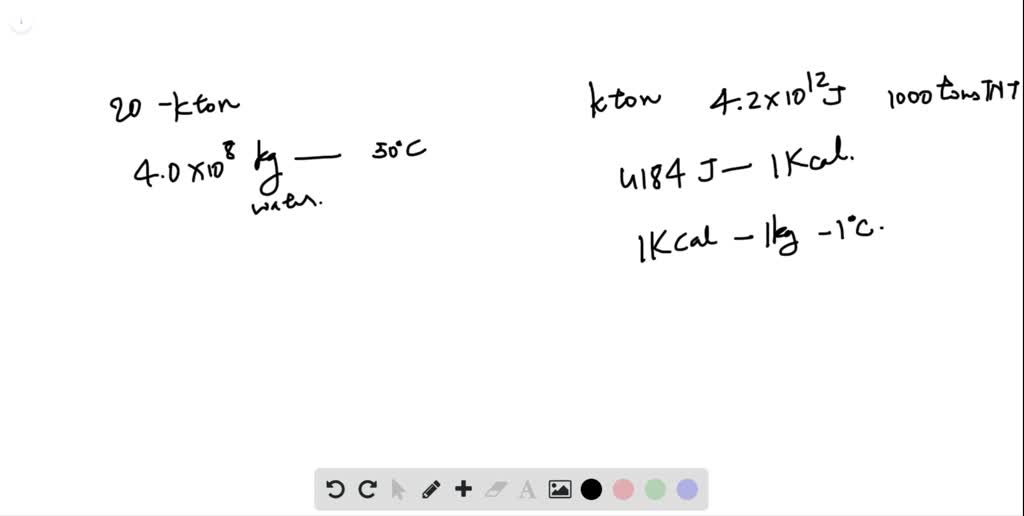
SOLVED:The kiloton, which is used to measure the energy released in an atomic explosion, is equal to 4.2 ×10^12 J (approximately the energy released in the explosion of 1000 tons of TNT).

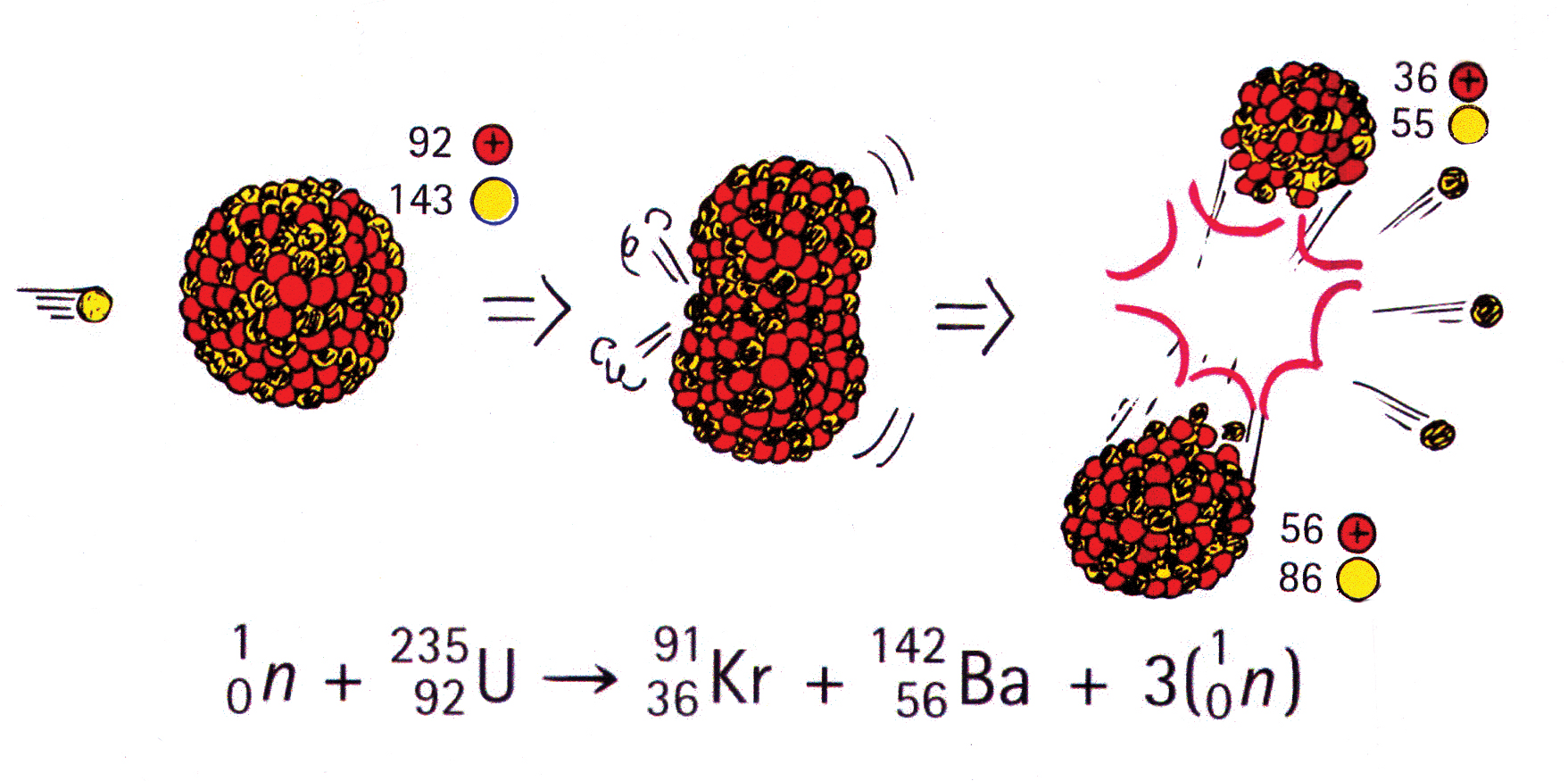
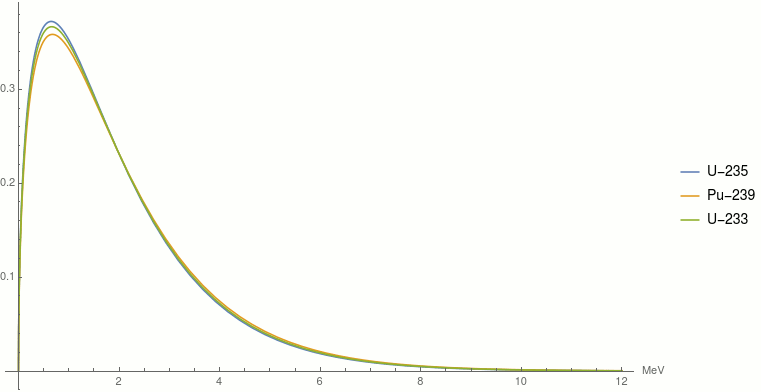
An explosion of atomic bomb releases an energy of 7.6xx10^(13)J. If 200 MeV energy is released on fission of one .^(235)U atom calculate (i) the number of uranium atoms undergoing fission. (ii)

Calculate the energy released by 1 g of natural uranium assuming 200 meV is released in each fission event and that the fissionable isotope ^23U has an abundance of 0.7
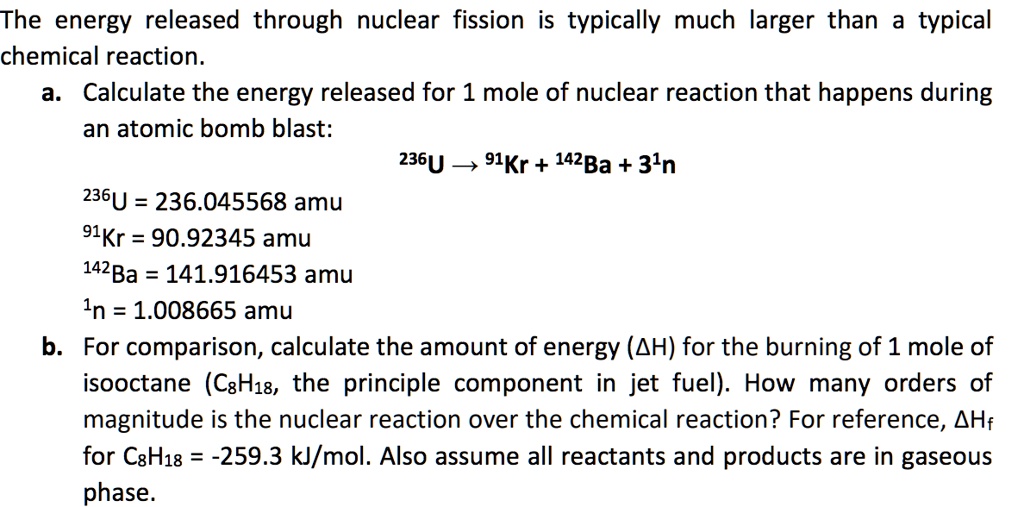
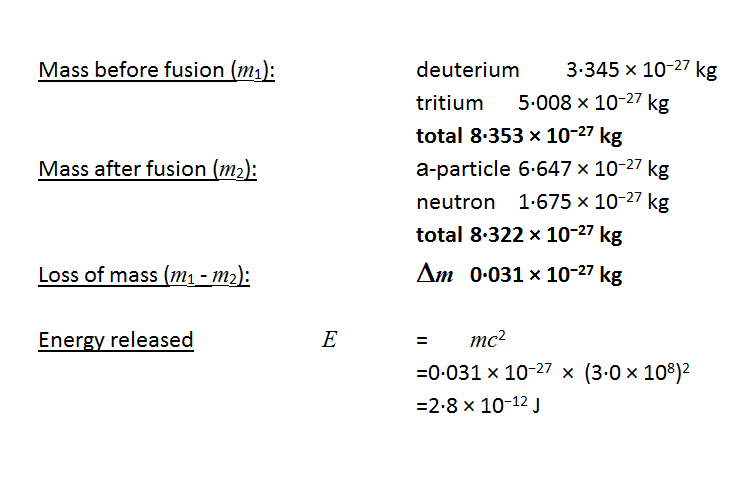
SOLVED: The energy released through nuclear fission is typically much larger than typical chemical reaction Calculate the energy released for 1 mole of nuclear reaction that happens during an atomic bomb blast:

For complete combustion of ethanol, C2H5OH(l) + 3O2(g)→ 2CO2(g) + 3H2O(l) , the amount of heat produced as measured in bomb calorimeter is 1364.47 kJ/mol at 25^C . Assuming ideally, the enthalpy