What is the pH of the resulting solution when the equal volume of 0.1M Noah and 0.01M HCL are mixed? - Quora

Calculate pH of the following solutions: (i) 0.001M HNO3 (ii) 0.005M H2SO4 (iii) 0.01M KOH (iv) 10^-8M NaOH (v) 0.0008M Ba (OH) 2
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community

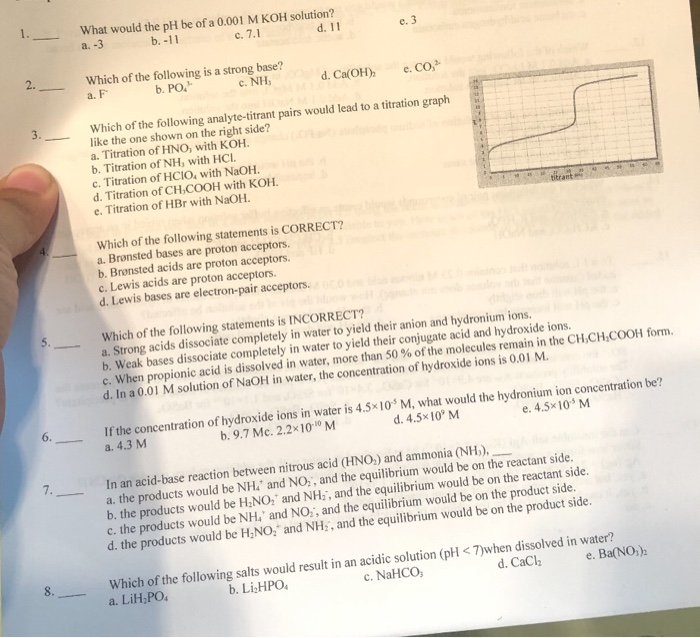
Assuming complete dissociation, calculate the pH of the following solutions:(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH

Calculate pH for: (a) `0.001 NaOH`, (b) `0.01N Ca(OH)_(2)`, (c ) `0.01M Ca(OH)_(2)`, (d) `10^(-8 - YouTube

Topic 18- Acids and bases 18.1 Calculations involving acids and bases 18.2 Buffer solutions 18.3 Salt hydrolysis 18.4 Acid-base titrations 18.5 Indicators. - ppt video online download