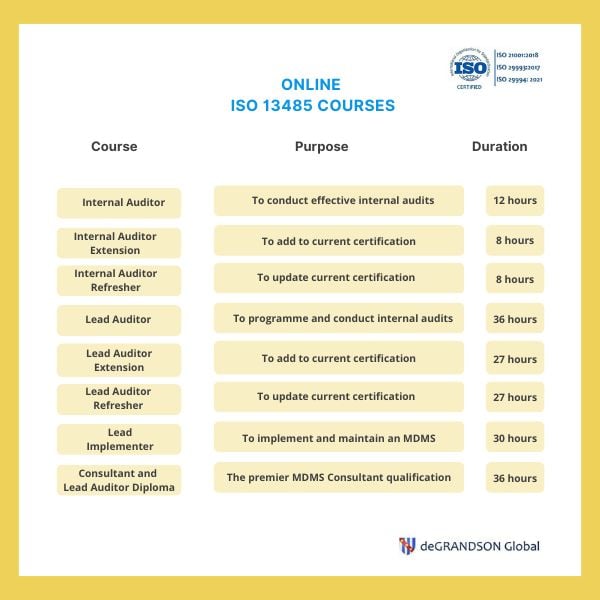
Lead Auditor Training & Certification on MD-QMS Requirements for Regulatory Purposes based on ISO 13485 | TÜV SÜD in India

Medical Device Quality Management System Auditor (ISO 13485) - Certus Professional Certification Inc.

Manufacturing And Product Development Support for Medical Devices / IVDs (Class C / D) -CliniExperts
IAF Mandatory Document Determination of Audit Time of Quality and Environmental Management Systems (IAF MD 5: 201X)